Congenital Heart Disease: A Simplified Review
José Hernández Jiménez
2/13/202511 min read
Congenital Heart Disease: A Simplified Review
Introduction
Congenital heart disease (CHD) is the most common congenital defect, affecting approximately 1% of newborns worldwide. It is a group of structural abnormalities of the heart and major blood vessels that originate during fetal development. These anomalies can compromise blood flow, leading to clinical consequences ranging from asymptomatic cases to severe heart failure.
Congenital heart disease represents one of the leading causes of infant mortality, although advances in diagnosis and treatment have improved the life expectancy and quality of life for many patients. Its origin is multifactorial, with a combination of genetic and environmental factors influencing its development. In this context, it is essential to understand the physiology of the heart, its adaptations during fetal life, and the abnormalities that lead to the disease.
Below, we will explore the main types of congenital heart diseases, their impact on blood circulation, and the risk factors involved in their onset
The heart is the central organ of the circulatory system and is composed of four chambers: two atria (right and left) and two ventricles (right and left). Its function is to pump oxygenated blood to the tissues and return deoxygenated blood to the lungs for reoxygenation. This process is regulated by four valves:
Mitral valve (bicuspid): Separates the left atrium from the left ventricle.
Tricuspid valve: Separates the right atrium from the right ventricle.
Aortic valve: Regulates the outflow of blood from the left ventricle into the aorta.
Pulmonary valve: Controls the flow of blood from the right ventricle into the pulmonary artery.
Additionally, the heart is made up of three layers: the pericardium (outer protective layer), the myocardium (muscle tissue responsible for contraction), and the endocardium (inner layer that lines the chambers and valves). Its function is regulated by an internal electrical system, where the sinoatrial node acts as the natural pacemaker, generating electrical impulses that control heartbeats.
The Heart and Circulation: Foundations for Understanding Congenital Heart Disease
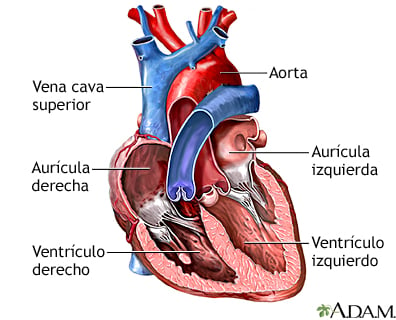
Figure: mEDLINEPLUS.GOV
During fetal life, blood circulation is different from that of adults, as oxygen and nutrients come from the placenta through the umbilical cord. To ensure efficient blood flow, there are three key vascular communications:
Ductus venosus: Directs oxygenated blood from the placenta to the fetal heart.
Foramen ovale: Allows blood to pass directly from the right atrium to the left atrium, bypassing the pulmonary circulation.
Ductus arteriosus: Connects the pulmonary artery to the aorta, facilitating systemic perfusion without the need for blood to pass through the lungs.
At birth, these fetal shunts progressively close, adapting circulation to extrauterine life. However, in some congenital heart defects, this process does not occur properly, which can lead to significant hemodynamic disturbances.
Fetal Circulation and Postnatal Adaptations
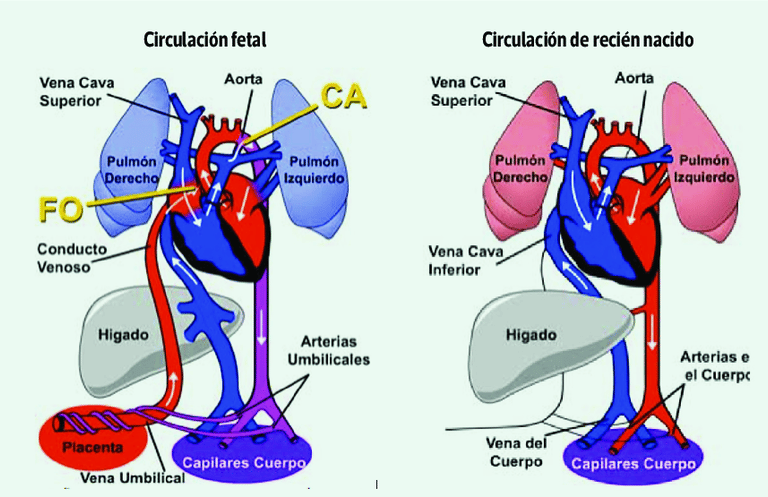
Figure: EL PULMÓN AL MOMENTO DE NACER. (2023). Neumología Pediátrica, 18(2), 32-36.
Congenital heart defects can be classified into two main groups based on the presence or absence of cyanosis, a bluish discoloration of the skin caused by low oxygen levels in the blood.
Cyanotic Heart Defects
These occur when deoxygenated blood mixes with oxygenated blood due to a right-to-left shunt, reducing the amount of oxygen available in the systemic circulation. The main cyanotic heart defects include:
Tetralogy of Fallot (TOF)
Transposition of the great vessels (TGA)
Tricuspid atresia
Common arterial trunk
Total anomalous pulmonary venous return
Acyanotic Heart Defects
In these conditions, the oxygenation of the blood is not significantly compromised, although there may be alterations in blood flow. Some examples include:
Ventricular septal defect (VSD)
Atrial septal defect (ASD)
Persistent ductus arteriosus (PDA)
Coarctation of the aorta
Aortic and pulmonary stenosis
Approximately 25% of CHD cases require surgical intervention during the first year of life, which poses a challenge in terms of diagnosis and treatment.
Classification of Congenital Heart Disease
The etiology of congenital heart disease (CHD) is complex and multifactorial. The main risk factors include:
Genetic Factors
Prevention through proper prenatal care and folic acid supplementation has been shown to be effective strategies to reduce the risk of some congenital heart diseases.
Congenital heart diseases are one of the leading causes of cardiovascular disease in childhood. Their origin may be determined by genetic, environmental, or a combination of both factors, highlighting the importance of early diagnosis and treatment.
Some congenital heart diseases are associated with chromosomal abnormalities and genetic mutations. Several conditions have been identified that predispose individuals to these defects, such as:
Down syndrome
DiGeorge syndrome
Marfan syndrome
Noonan syndrome
Turner syndrome
Maternal Environmental Factors
The intrauterine environment plays a key role in heart development. Some maternal factors that may influence the development of CHD include:
Folic acid deficiency
Alcohol, tobacco, or drug use during pregnancy
Maternal infections (such as rubella or cytomegalovirus)
Poorly controlled maternal diabetes
Thanks to advancements in pediatric cardiology, most children with CHD can receive surgical and medical interventions that improve their prognosis. However, challenges still exist in early detection and access to specialized treatments, especially in countries with limited resources.
Ongoing studies on cardiac developmental biology and genetics will provide a better understanding of these pathologies in the future and allow for the design of more effective strategies for prevention and clinical management.
Risk Factors and Causes of Congenital Heart Disease
Figure: Woods et al. Factor von Willebrand y Enfermedad de von Willebrand: nuevos enfoques diagnósticos; Federación Bioquímica de la Provincia Buenos Aires; Acta Bioquímica Clínica Latinoamericana; 50; 2; 6-2016; 273-290
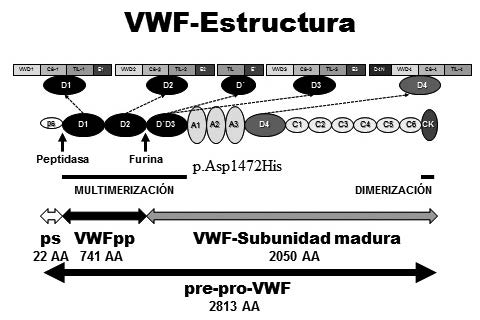
Early diagnosis is crucial in congenital heart disease, which is why much research focuses on identifying biomarkers and biochemical parameters that can predict postoperative complications. Some of these biomarkers include serum lactate, NT-ProBNP, PaO₂, serum creatinine, serum albumin, and glucose. Analyzing these markers can provide valuable information about patient progress, helping prevent complications and improve clinical outcomes.
For example, elevated serum lactate levels indicate a deficiency in oxygen supply to tissues, which can delay recovery after surgery. Similarly, NT-ProBNP, a hormone released by the heart in response to stress, is associated with higher mortality and the need for inotropic support. In contrast, low serum albumin levels can be an indicator of malnutrition, which prolongs hospitalization and affects cardiac recovery.
Another important factor in prognosis is the preoperative activation of von Willebrand factor (VWF), which is related to a higher risk of postoperative thrombosis. These findings reinforce the need for prospective and retrospective studies to determine which biomarkers have the greatest predictive value in patients with congenital heart disease.
Importance of Biomarkers and Genetic Testing in the Diagnosis and Prognosis of Congenital Heart Disease
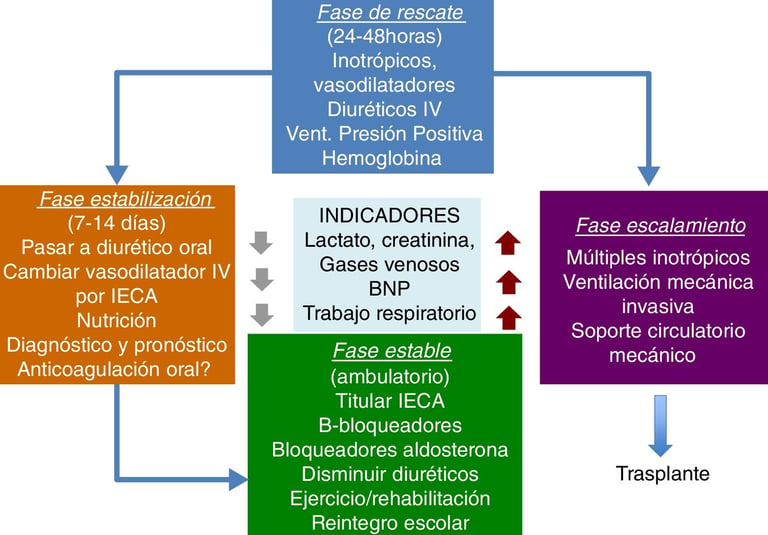
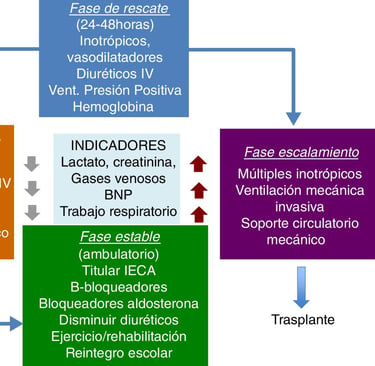
Figure: Cassalett-Bustillo. Falla cardíaca en pacientes pediátricos. Fisiopatología y tratamiento. Parte II. 2018. Revista Colombiana de Cardiología
Given that congenital heart defects affect approximately 1 in 110 live births, advances in prenatal diagnosis have been crucial in improving clinical outcomes. Fetal echocardiography allows for a detailed evaluation of cardiac anatomy and function before birth, facilitating the planning of personalized perinatal management strategies.
A multidisciplinary approach, including fetal cardiologists, neonatologists, and maternal-fetal medicine specialists, is essential to ensure timely and coordinated treatment. Patel et al. have shown that fetal echocardiographic characteristics can predict the severity of neonatal hemodynamic instability, enabling more precise interventions.
Among the most innovative diagnostic techniques is the acute maternal hyperoxia test (AMH), which evaluates the fetal response to inhaled oxygen. This test, typically performed after 34 weeks of gestation, can help predict postnatal physiology in complex congenital heart defects.
Importance of Genetic Testing in Congenital Heart Disease
Genetic testing has proven to be a key tool in identifying the underlying cause of many congenital heart diseases. These tests not only allow for a more accurate diagnosis but also help detect associated comorbidities, facilitate reproductive planning, and optimize treatments. However, its use remains limited: only 55% of newborns with surgical congenital heart disease undergo these tests, despite a diagnostic yield of 44%.
Among the most commonly used genetic tests are:
Chromosomal analysis:
This test identifies chromosomal abnormalities such as trisomies (e.g., trisomy 21 in Down syndrome, trisomy 18) and deletions. The 22q11.2 deletion syndrome, identified in 1981, is one of the most common variants in patients with congenital heart disease.Chromosomal microarray (CMA):
It detects copy number variants (CNVs) with greater precision than karyotyping, allowing for the diagnosis of conditions such as the 1p36 deletion and 6q25.1 deletion, both associated with congenital heart disease and cardiomyopathies.Exome sequencing:
This analyzes the protein-coding genes of the DNA, covering about 1-2% of the genome, and has identified variants in genes such as MYBPC3 (locus 11p11.2), associated with cardiomyopathies. Its use has led to diagnosis in up to 33% of cases of familial congenital heart disease.Whole genome sequencing:
It examines all nuclear DNA, including non-coding regions involved in transcriptional regulation. This technique is superior to exome sequencing and has identified pathogenic variants in 27-46% of patients with congenital heart disease and cardiomyopathies.Mitochondrial genome sequencing:
This detects alterations in mitochondrial DNA, crucial for understanding heart diseases related to the heart's energy metabolism. However, the variability in mutational load across different tissues makes its analysis more complex.
Challenges and Future Perspectives
Despite advances in genetic testing, variants of uncertain significance continue to pose a clinical challenge. Some rare mutations in genes such as SNIP1 (locus 1p34.1) and SHROOM3 (locus 10q22.1) have recently been associated with heart diseases, but their relevance is still under investigation.
The use of artificial intelligence and natural language processing has improved the interpretation of genetic data, but standardized approaches are still needed to enhance diagnostic accuracy.
In the future, more clinical and translational research will be required to better understand the genetic mechanisms of congenital heart diseases and optimize diagnostic and treatment strategies.
Early and accurate diagnosis of congenital heart diseases is crucial for improving clinical outcomes. Biomarkers such as serum lactate, NT-ProBNP, and serum albumin are useful tools for predicting postoperative complications, while genetic testing helps identify underlying causes and design personalized therapeutic strategies.
Although genetics has revolutionized the diagnosis of these diseases, its clinical application remains limited. The implementation of multidisciplinary and integrated approaches is essential for optimizing the management of patients with congenital heart disease and improving their long-term prognosis.
Advances in Prenatal Diagnosis
Figure: medicalexpo.es
Treatments
Surgical procedures have been fundamental in the management of patients with severe congenital heart defects, especially those diagnosed with tricuspid atresia, pulmonary atresia, aortic coarctation, and Tetralogy of Fallot (TOF). These treatments include the use of prosthetic grafts, mechanical valves, and other devices made from materials such as Dacron, GORE-TEX® (poly(tetrafluoroethylene - PTFE)), among others. However, these materials present several disadvantages, such as limited durability, difficulties in growth and remodeling, risk of thrombosis, hemorrhagic effects due to anticoagulation, calcification, endocarditis, and other problems.
Despite these limitations, short-duration prosthetic grafts have been developed, requiring multiple surgical interventions throughout the patient's life. The most common materials include Dacron, mechanical valves, and GORE-TEX® grafts, as well as decellularized xenografts and homografts (usually from porcine and bovine origins). While these materials are durable, their use can generate adverse immune responses, collagen degradation, and long-term dependency on anticoagulation, with the associated bleeding risk. Additionally, they face issues such as limited availability, susceptibility to calcification, and limited growth or in vivo remodeling capacity.
Although autografts offer advantages such as a lower risk of immune response and a better hemodynamic profile, their availability is limited, and they are prone to dilation, which can cause valve regurgitation. On the other hand, Dacron and GORE-TEX® are readily available materials but present significant challenges, especially when used in procedures such as the extracardiac Fontan to treat patients with TOF. In these cases, a high incidence of stenosis is observed when using Dacron grafts, and there is a surgical delay in neonates until the inferior vena cava reaches a diameter comparable to its final adult size due to the patient's somatic overgrowth. Given the aforementioned limitations, alternative options have been explored, designed to minimize the need for multiple surgical interventions and improve patients' quality of life.
Patients with congenital heart disease (ACHD) and left ventricular (LV) systemic failure are typically treated with medications similar to those used in acquired heart failure. These include beta-blockers, renin-angiotensin system inhibitors (such as ACE inhibitors, ARBs, or angiotensin-neprilysin inhibitors), mineralocorticoid receptor antagonists, sodium-glucose cotransporter 2 inhibitors, diuretics, and even combinations of hydralazine and nitrates. However, because these patients have historically been excluded from large clinical trials, evidence on the effectiveness of these treatments in ACHD is limited.
In patients with right ventricular (RV) systemic failure, the use of beta-blockers such as carvedilol and metoprolol has shown some benefits in ventricular function and exercise tolerance, although without a clear impact on ejection fraction. Other treatments, such as losartan and valsartan, have shown mixed results in improving right ventricular function, while ACE inhibitors have not demonstrated significant benefits in exercise capacity or ejection fraction.
In patients with Fontan circulation, the use of pulmonary vasodilators like sildenafil, bosentan, and iloprost has been explored, with variable effects on cardiovascular function and exercise performance. Some studies suggest that endothelin receptor antagonists may help reduce pulmonary vascular resistance, especially in those with elevated levels. For Eisenmenger syndrome, treatment focuses on supportive measures, symptom control, and the use of pulmonary vasodilators when appropriate, although any medication that significantly lowers blood pressure and worsens cyanosis should be avoided. In severe cases, heart and lung transplantation remains an option.
The management of ACHD with heart failure requires individualized evaluation, considering the anatomical and physiological particularities of each patient. Monitoring and medication effectiveness in ACHD present challenges due to the complexity of these conditions. The response to medications can vary significantly, and in some cases, doses must be adjusted based on parameters such as renal function. Additionally, patients with ACHD may have greater frailty, which can influence their tolerance to treatment. In cases of advanced heart failure, hospital management may require the use of vasoactive drugs such as norepinephrine and milrinone to stabilize heart function. As these patients age and develop progressive symptoms of heart failure, advanced therapies, such as mechanical circulatory support or heart transplantation, should be considered to improve clinical outcomes.
Pharmacological Treatment
Sinus node dysfunction can be congenital or a complication of previous surgeries. It is observed in patients with congenital heart abnormalities, such as left atrial isomerism or juxtaposition of the left side of the atrial appendages. Additionally, it can arise after procedures such as Fontan surgery, atrial switch, or the repair of tetralogy of Fallot. Similarly, atrioventricular (AV) block can be congenital or develop after surgical interventions, such as the repair of atrioventricular septal defects or valve surgeries. In cases where bradycardia or AV block affects the quality of life or cardiac function, pacemaker implantation is recommended. Some studies have shown that in patients with congenital heart disease who rely heavily on ventricular pacing, a pacemaker-associated cardiomyopathy may develop, although without severe complications associated with the use of leadless pacemakers.
Sudden cardiac death is one of the leading causes of death in patients with congenital heart disease. To prevent this, implantable cardioverter-defibrillators (ICDs) are indicated for individuals with left ventricular dysfunction with an ejection fraction ≤35%, a history of unexplained syncope with suspected arrhythmia, or with risk factors such as ventricular tachycardia and widened QRS complex. Patients with repaired tetralogy of Fallot, right ventricular dysfunction, or Eisenmenger syndrome with good functional status may benefit from these devices. ICDs also enable remote monitoring of atrial arrhythmias, facilitating adjustments in antiarrhythmic therapy. The choice of implantation method, whether transvenous or epicardial, depends on the patient's congenital anatomy and previous surgeries.
Cardiac resynchronization therapy (CRT) is an option to improve heart failure in individuals with congenital heart disease who have ventricular dysfunction and delayed electrical conduction in the heart. CRT has been shown to improve functional capacity, reduce QRS duration, and increase ejection fraction, with sustained long-term benefits. Even in older patients with complex cardiac anatomies, the success rate is similar to that observed in individuals with acquired heart disease. Furthermore, studies have demonstrated that CRT reduces mortality compared to patients with ischemic or non-ischemic cardiomyopathy. Cardiac conduction system stimulation, a more physiological alternative, has also shown significant improvements in ventricular function, consolidating CRT as a key strategy for managing heart failure in patients with congenital heart disease.
Treatment through the use of medical devices
References:
Pidaparti M, Geddes GC, Durbin MD. Clinical Genetic and Genomic Testing in Congenital Heart Disease and Cardiomyopathy. J Clin Med. 2024 Apr 26;13(9):2544.
Francisco-Pascual J, Mallofré Vila N, Santos-Ortega A, Rivas-Gándara N. Tachyarrhythmias in congenital heart disease. Front Cardiovasc Med. 2024 Jun 3;11:1395210.
Salih T, Caputo M, Ghorbel MT. Recent Advances in Hydrogel-Based 3D Bioprinting and Its Potential Application in the Treatment of Congenital Heart Disease. Biomolecules. 2024 Jul 18;14(7):861.
Meng X, Song M, Zhang K, Lu W, Li Y, Zhang C, Zhang Y. Congenital heart disease: types, pathophysiology, diagnosis, and treatment options. MedComm (2020). 2024 Jul 5;5(7):e631.
Carazo M. Medical Therapy for Heart Failure in Adult Congenital Heart Disease Patients. Struct Heart. 2024 Apr 10;8(4):100297.
Zhou Shifan , Liu Lu , Jin Xiaochuang , Dorikun Daniel , Ma Songfeng. Biomarkers predicting postoperative adverse outcomes in children with congenital heart disease: a systematic review and meta-analysis. Frontiers in Pediatrics. 2025. 13
